The small spotlike organizer of radial-symmetric animals enlarged to a huge ring in vertebrates
Axes formation must be a self-organizing process although maternal asymmetries might play an important role. An example is the generation of the complete set of body axes, starting with a single cell isolated from an eight-cell mouse embryo, Below schematic drawings are given that illustrates the steps in the generation of a near-Cartesian coordinate system, starting from a sphere and using a frog-like development as example. First a separation of the ectoderm (blue) and endoderm (brown) occurs along the animal-vegetal axis (animal / anterior is up). Mesoderm (green) becomes induced at the ecto/endoderm border. The known components of the endo/mesodermal patterning suggest that a genuine pattern forming process is involved. Both endoderm and mesoderm invaginate (the yolk is ignored in the drawing below); the side of invagination has the geometry of a large ring, the blastopore.
After invagination of the endoderm, the animal-vegetal axis no longer exists. After invagination the formerly antipodal poles are adjacent to each other. They are no longer appropriate to generate positional information. Thus, the anteroposterior axis needs a different signalling system for its organization although it has the same spatial orientation. The blastopore (marginal zone, green) becomes the organizing region for the AP axis and is posterior. This early gastrula forms essentially the brain and the heart and is proposed to be homologous to the body pattern of the hydra.
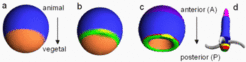
The formation of the spot-like Spemann-type organizer (dark green/yellow) responsible for the dorso-ventral pattern is restricted to the blastopore. Thus, since the blastopore became a large ring , the dorsal organizer has automatically a pronounced off-axis position as required. The Spemann-type organizer with its spot-like extension is by itself insufficient to generate a dorsoventral pattern along the long-extended anteroposterior axis. An important step is, therefore, the generation of a midline organizer.
It is the marginal ring and not the Spemann-Organizer that provide the positional information for the AP patterning. Evidence exists that WNT signals, generated in the ring, are involved in the posterior transformation of the more anteriorely located cells that form the future brain [1,2]. Also the activation of more posterior HOX genes takes place in the ring except of the organizing region [3]. For the zebra fish it has been shown that AP-markers appear in the correct order even in the complete absence of a dorsal organizing region [4]. The details of the AP specification support the view that that the AP axis is generated in two parts. The ancestral anterior part (brain and heart) occurs by by posterior transformation under the influence of a morphogen signal. In contrast, the more posterior part is patterned sequentially. An oscillation generates the periodic somites and the sequential pattern of HOX-gene activation.
The enlargement of the posterior organizer to a large ring is a specific feature of vertebrates. In spiders, for instance, the blastopore remains a small spot while the anterior pole enlarged to a ring (below left) [5]. Likewise in Drosophila, at the critical stage the oocyte has the shape of a cone. The tip is posterior, the circular antipodal side anterior. The movement of the nucleus underneath the membrane is necessarily connected with a symmetry breaking [6].

Further Reading and References
Meinhardt, H. (2004). Different strategies for midline formation in bilaterians. Nat Rev Neurosci 5,502-510 [PDF]
- Kiecker, C. & Niehrs, C. (2001). A morphogen gradient of wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189-4201.
- Dorsky, R.I., Itoh, M., Moon, R.T. & Chitnis, A. (2003). Two tcf3 genes cooperate to pattern the zebrafish brain. Development 130, 1937-1947.
- Wacker, S.A., McNulty, C.L. & Durston, A.J. (2004). The initiation of Hox gene expression in Xenopus laevis is controlled by Brachyury and BMP-4. Dev. Biol. 266, 123-137.
- Ober, E.A. & Schulte-Merker, S. (1999). Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev. Biol. 215, 167-181.
- Akiyamada-Oda, Y. and Oda, H. (2003). Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disk epithelial cells. Development 130, 1735-1747
- Roth, S. (2003). The origin of dorsoventral polarity in Drosophila. Phil. Trans R Soc Lond B 358, 1317-1329.

