Protein Folding, Unfolding and Degradation
Jörg Martin
A protein´s proper folding is required to arrive at a functional and active state. At the end, a protein’s fate is degradation, typically preceded by dissociation of oligomeric complexes and unfolding of the polypeptide chain. Using a variety of biochemistry, biophysics and microbiology techniques, we focus on prokaryotic model systems to better understand these intricate processes.
The biochemistry of molecular chaperones and AAA ATPases
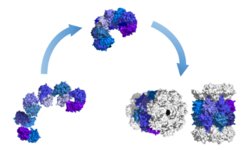
Protein folding is continuing to be an active field of research. Folding in the cell is assisted by molecular chaperones, whereas AAA ATPases participate in the unfolding of proteins as a prerequisite for protein degradation. We investigate the properties of selected bacterial and archaeal chaperonins and AAA ATPases to arrive at an understanding of their mechanism of action.
The evolution of ubiquitination and the proteasome
We actively study an ancestral archaeal ubiquitination system and prokaryotic versions of the proteasome. Our primary goal is to learn about protein degradation pathways in these organisms. On the long run, we aim to understand how different protein folding and degradation machineries evolved in bacteria and archaea.
