Graded cues and growth cone navigation (e.g. in the retinal-tectal and the olfactory system)
A particularly interesting aspect of development is the generation of the neural network. The mechanisms must be such as to allow a limited set of genetic determinants to specify a much larger number of connections. A prototypical example is the mapping of one area of the nervous system onto another in the visual system. Sperry (1963) [1] has proposed that target positions are specified by graded substances in target tissue interacting with substances on axons graded with respect to position of their origin. For a long period, gradient mechanisms were mostly conceived in terms of differential adhesion, whereas attention is now focussing on intra-growth cone processing of graded signals.An analysis of this process led to the following criteria and conditions to be incorporated into the models (Gierer 1981b , 1987 ):
- Aside from the increasing, impressive evidence on chemical identification of graded molecules involved (see also Bonhoeffer homepage ), it is the capability of axons for approaching the target position from different aspects in a two-dimensional field which is per se a strong indication for the involvement of gradients.
- Targeting within the target field requires counter-graded effects, either by antagonistic gradients, or by a single gradient in each dimension exerting attractive effects at low, reverting to inhibitory (repulsive) effects at high concentrations. The intriguing one-gradient-per-dimension model for growth cone navigation is somewhat analagous to the role of longitudes and latitudes in ocean navigation, and to the specifications of seats by row number and number-in-the-row, allowing for directional rather than random approach from any point of entry. A further requirement for mapping is the modulation of the counter-graded effects by components of the growth cone itself which depend on the origin of the corresponding axon. In this way, different axons can be guided to different target positions. Recent evidence, reviewed by Song and Poo [2] demonstrates modulating effects within growth cones on their direction of growth, including reversions from attraction to repulsion.
- Subtle transduction and processing of graded signals in the navigating growth cones are proposed to be strongly enhanced by intra-growth- cone pattern formation, in analogy to directional sensing in the course of chemotactic movement (see Orientation of chemotactic cells and growth cones ).
- Empirical evidence on chemotactic movement indicates that about 1% concentration difference across a cell diameter suffice for reliable directional guidance.
- Branching may be modeled for on the assumption that branching occurs if the forward direction is sensed as entirely wrong. In particular, this mechanism may account for extensive branching near the target site, leading to the terminal arbor.
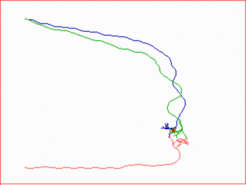
Computer simulation showing the main features of guidance by gradients: the approach of the target position from different aspects including course corrections en route and the formation of the terminal arbor.
Regulatory features, such as compression of maps, can be incorporated into the models (Gierer, 1983).Thus, if growth cones are assumed to induce, in an unspecific manner, a repellent substrate in the tectum, this simple mechanism would lead to expansion or contraction of the map, eventually occupying the tectal space available while fully maintaining the topographic order.
The theory may apply not only for mapping in the visual but also in the olfactory system (Gierer, 1998). There, axons of any given olfactory receptor cell type converge on a given area of the olfactory bulb. It is proposed that, just as in the visual system, again all axons may use the same type of simple graded cues across the target tissue as coordinate system of growth cone navigation irrespective of the target positions. The subtlety and specificity of directional sensing resides in the navigating growth cone. In the olfactory system, it depends not on position of origin, but on receptor cell type. Following targeting at approximately correct positions, specific molecular interactions as well as activity-dependent processes are expected to lead to map sharpening and fine tuning (for review see Gierer and Müller, 1995 )
Examples of mathematical models of the two– as well as the one-gradient-per-dimension case are given in Gierer, 1987, Fig. 1 and Fig. 2, respectively.

A simple general version based on one gradient for each of the two dimensions of the target field (Gierer 1998) on which the computer simulation shown above is based, is to assume transduction and processing of gradients f(x) and g(y), sensed by the navigating growth cone in the area of contact with target tissue, leading to an intra-growth-cone-distribution of the product of processing
p(x,y) = αf(x)/ (1 + [αf(x)]2 ) + βg(y) / (1+ [ βg(y)]2 )
The p gradient is enhanced within the growth cone resulting in a focus of activity directing further growth of the axon towards the position of optimal value p, given by the coordinates x,y where f(x) = 1/α and g(y) = 1/β. α, β are the growth cone features defining the target position. For models of topographic projections as they occur in the visual system, α and β must depend monotonically on the coordinates of origin of the corresponding axons; in case of the olfactory system, α, β is assumed to depend on the receptor-cell-type, thus allowing for the convergence of the fibres of each receptor-cell-type towards the position of the corresponding glomerulus in the olfactory bulb.
The one-gradient-per-dimension model implies targeting towards a position determined by absolute values of the guiding gradients f and g. This corresponds formally to a quantitative matching behavior between features of growth cones and target tissue. However, in terms of mechanisms, the process is attributed to molecular kinetics of signal transduction and processing and not just to adhesion of complementary surface molecules which would be, in itself, insufficient for directional guidance. For models based on two antagonistically acting gradients in each dimension, it is their slopes, not just their absolute values that define the target position. The one-gradient-models are more elegant but it is prudent to remain open minded to multiple gradients as well since biological mechanisms are determined by evolution rather than by mathematical elegance.
Recent experimental findings (2006) in favor of our concepts on axonal guidance by gradients are referred to in an “editors summary” with respect to the visual system in Nature [3], and in a reviewing article by Chen and Flanagan with respect to the olfactory system in Cell [4].
Further Reading and References
Gierer, A. (1987). Directional cues for growing axons forming the retinotectal projection. Development 101, 479-489. [PDF]
- Sperry, R.W. (1963). Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad Sci. USA 50, 703-710.
- Song, H.J. and Poo, M.M. (1999). Signal transduction underlying growth cone guidance by diffusible factors. Cur. Opin. Neurobiol. 9, 355-363.
- Editors summary, One in the eye, Nature 439, Jan. 05 (2006)
- Y. Chen and J. G. Flanagan, Follow your nose: Axon pathfinding in olfactory map formation. Cell 127, 881-884 (2006)

