Beta-catenin and WNT expression in hydra show features expected for the activator
By searching for molecules that are known to play a decisive role in organizer formation in higher organisms, homologues of the Hy-beta-catenin, wingless and Tcf (Hobmayer et al., 2000) [1] as well as Brachyury [2] have been found. All these genes are expressed in the head region, the expressions of Hy-beta-catenin and Hy-Wnt are shown in the figure below.
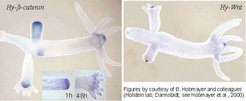
Upon regeneration these molecules behave as expected from our theory: the activity reappears in the region were the regenerating head is expected, a process that requires about 1-2 hours (inserts in the left figure above [1]). Moreover, ectopic expression of Hy-beta-catenin RNA in Xenopus can induce a secondary embryonic axis, indicating that the crucial pathways for organizer formation are well preserved in evolution.
Although all these genes are expressed in the head region, there are differences in the expression pattern: Hy-beta-catenin and Hy-Tcf (above left) have a somewhat wider distribution. During bud formation, these are distributed over the entire bud in a graded fashion. Later, the high levels become more restricted to the head region. In contrast, Hy-wnt is from the beginning more confined to the surrounding of the opening of the gastric column. Thus, both systems have different wavelength. These different expression patterns suggest that these molecules are not just part of a simple auto regulatory loop.
After dissociation into single cells and re-aggregation, Hydra cells can form complete animals [4]. During this process Hy-beta-cat/Tcf on the one hand and Hy-Wnt on the other behaves differently. Taking cells from the gastric column that don’t express these genes, Hy-beta-cat / Tcf become first active in a homogeneous way. Later, isolated local concentrations emerge that eventually give rise to head formation. In contrast, Hy-Wnt activity emerges directly in very sharp spots that form later the oral opening [1]:
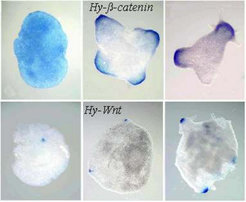
Figures by courtesy of B. Hobmayer and colleagues (T. Holstein lab, Darmstadt; see Hobmayer et al.[1] )
This suggests that first relative smooth Hyß-cat/Tcf distributions are formed by a genuine patterning process (as expected from our general model). The highest levels of these peaks are used to trigger the Hy-Wnt activity. This behavior can be reproduced in simulations assuming that a relatively high beta-catenin concentration is the prerequisite to trigger the Wnt-activation.
Taking together, this suggests that at least three systems are involved in the formation of the head region: (i) the primary system in which Hy-beta-catenin and Hy-TCF signaling is involved. (ii) A second but closely linked system leads to a sharp signal Hy-Wnt at the oral opening. It is triggered by higher levels of ß-batenin. (iii) The third system is smoothly graded over the whole axis and has a long time constant. It provides the graded competence, necessary to maintain a single organizing region. Its molecular nature is unknown but it can be influenced by diacyl-glycerol (DAC).
While arguments can be given that Wnt has an auto regulatory element, no molecule has been found that has the characteristic properties of the inhibitor. Whether the inhibitory signal spreads by diffusion or other mechanisms is unknown. However, by a series of intriguing experiments based on the regeneration of hydras from composite cell aggregates [3], the ranges of activating an inhibitory effects have been determined. A ratio of both of about 20 has been found, a value almost identical we have used in our simulations.
References
- Hobmayer, B., Rentzsch, F., Kuhn, K., Happel, C.M., Cramer von Laue, C., Snyder, P., Rothbacher, U. and Holstein, T.W. (2000). Wnt signaling molecules act in axis formation in the diploblastic metazoan hydra. Nature 407, 186-189.
- Technau, U. and Bode, H.R. (1999). HyBra1, a Brachyury homologue, acts during head formation in Hydra. Development 126, 999-1010.
- Technau U., von L.C., Rentzsch F., Luft S., Hobmayer B., Bode H.R. and Holstein T.W. (2000). Parameters of self-organization in hydra aggregates. PNAS 97,12127-12131.
- Gierer, A., Berking, S., Bode, H., David, CN., Flick, K., Hansmann, G., Schaller, H. and Trenkner, E. (1972). Regeneration of hydra from reaggregated cells. Nature New Biology 239, 98-101. [PDF]


